TRANSFORMING SERVICE INTO SOLUTIONS
THERAKOS® ECP EDGE IS A COMPREHENSIVE RANGE OF PRODUCT SUPPORT SERVICES TO HELP HEALTHCARE PROFESSIONALS (HCPs) DELIVER OPTIMIZED EXTRACORPOREAL PHOTOPHERESIS (ECP) IMMUNOMODULATION TO PATIENTS.
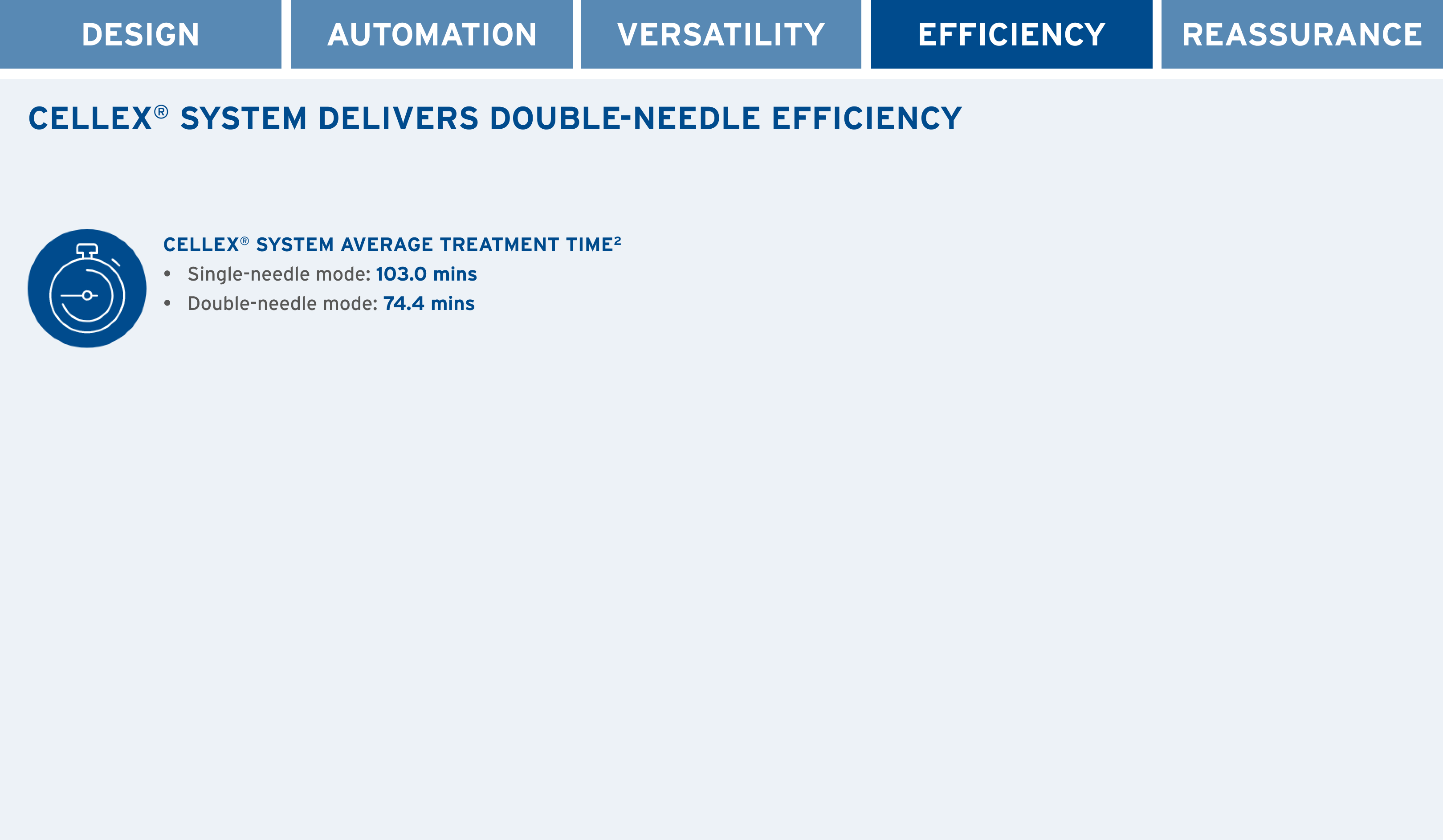
PERFORMANCE
- The CELLEX System represents our latest advancement in integrated ECP immunomodulation technology
- The CELLEX System is a device that we’re committed to improving according to your evolving needs. It’s why we make regular updates to the system; all designed to improve user experience and provide optimal delivery of ECP therapy
FLEXIBILITY AND ADAPTABILITY FACILITATE SERVICE PROVISION
- The CELLEX System offers beneficial operational features:
- A mobile, compact system that is easily moved/transported within the unit*
- Choice of single- or double-needle mode according to venous access conditions, and the ability to switch between the two at any time during the procedure1,2†
- Troubleshooting wizards to support efficient solutions for operators during ECP procedures
*Please inform your key account manager of device location changes within your unit prior to relocating the device. †Operators should use their medical judgment when deciding whether to administer treatment using the single- or double-needle mode, taking into account each patient’s needs and the instructions set forth in the Operator’s Manual.
VALIDATED TECHNOLOGY
- With full integration comes the reassurance of a completely sterile and continuous process1,3,4
-
The patient remains connected to the CELLEX System throughout the procedure. This is designed to:
- Minimize reinfusion errors or cross-contamination4
- Minimize risk of microbial contamination4
PATIENT & CUSTOMER FOCUS

COMPREHENSIVE DEVICE OPERATIONAL TRAINING
- On-site initial device training for nurses and other HCPs
- Periodic on-site device training to help HCP teams become more confident
- Ongoing on-site procedural device support, including training for specific patient needs
- Continuous improvement based on customer feedback
THERAKOS INSTITUTE ONLINE
- Offers a range of learning and self-study training opportunities, including:
- Access to training sessions on ECP and immunomodulation
- Access to all operator training resources for optimal patient care
THERAKOS® CELLEX® IMMUNOMODULATION
WHAT IS THERAKOS CELLEX IMMUNOMODULATION?
Extracorporeal photopheresis (ECP) is an immunomodulatory therapy that has shown efficacy in patients with the skin manifestation of cutaneous T-cell lymphoma and in system sclerosis5
HOW IS THERAKOS CELLEX IMMUNOMODULATION DELIVERED?
- THERAKOS CELLEX Immunomodulation is delivered via a closed, integrated, and automated ECP platform, the THERAKOS CELLEX Photopheresis System1,3,4
For more information on adverse events and warning and precautions, please refer to the Important safety Information for THERAKOS photopheresis systems.


WHAT ARE THE CLINICAL APPLICATIONS OF THERAKOS® CELLEX® IMMUNOMODULATION?
DOSING FOR PATIENTS WITH SKIN MANIFESTATIONS OF CTCL WHICH CAN BE ADJUSTED BASED ON PATIENT RESPONSE
The THERAKOS CELLEX Photopheresis System is used for the palliative treatment of the skin manifestations of cutaneous T-cell lymphoma (CTCL) and systemic sclerosis (SSc). Only health care professionals with training in THERAKOS Photopheresis should administer this therapy.
NORMAL TREATMENT FREQUENCY1
The patient should receive treatment on two successive days each month for six months.1 An “adequate response” is considered to be a 25% improvement in the skin score for patients with skin manifestations of cutaneous T-cell lymphoma and a 15% improvement in the skin score for patients with systemic sclerosis (see medication package insert for skin score determination) obtained within the first eight treatments and maintained for at least four weeks.1,7 Continued treatments may or may not lead to improvements of greater than 25% and 15%, respectively.1,7
ACCELERATED TREATMENT FREQUENCY1
Patients who fail to show an “adequate response” to treatment after eight treatments may have their treatment schedules increased to two successive days every two weeks for the next three months.1
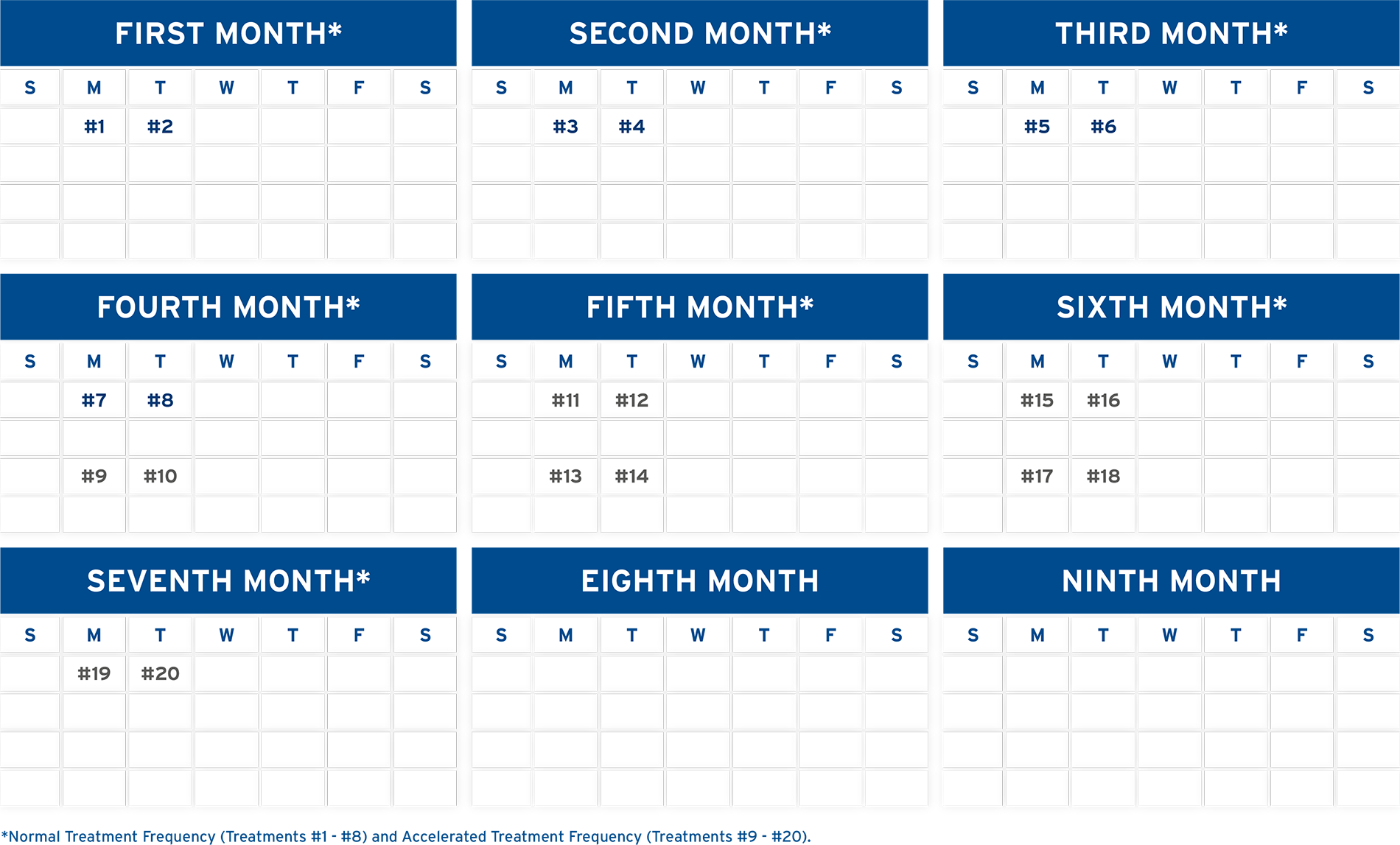
If no response, or less than an “adequate response” is achieved after 20 treatments, use clinical judgment to decide if photopheresis should be continued.1 In the clinical trial of methoxsalen (20 mcg/mL), 15 of 17 responses were seen within six months of treatment. Two patients did respond to treatment after six months.1
MAINTENANCE FREQUENCY1
Patients who attained a positive response in the clinical studies were maintained at a schedule of two consecutive days of treatment every five weeks for at least three months.1 When initial response is maintained at this schedule, photopheresis frequency may be gradually decreased, and in some cases may be discontinued entirely. However, the physician should continue to follow the patient on a regular basis.1
PHYSICIANS SHOULD DETERMINE THE APPROPRIATE TREATMENT SCHEDULE BASED ON THEIR PATIENT’S NEEDS
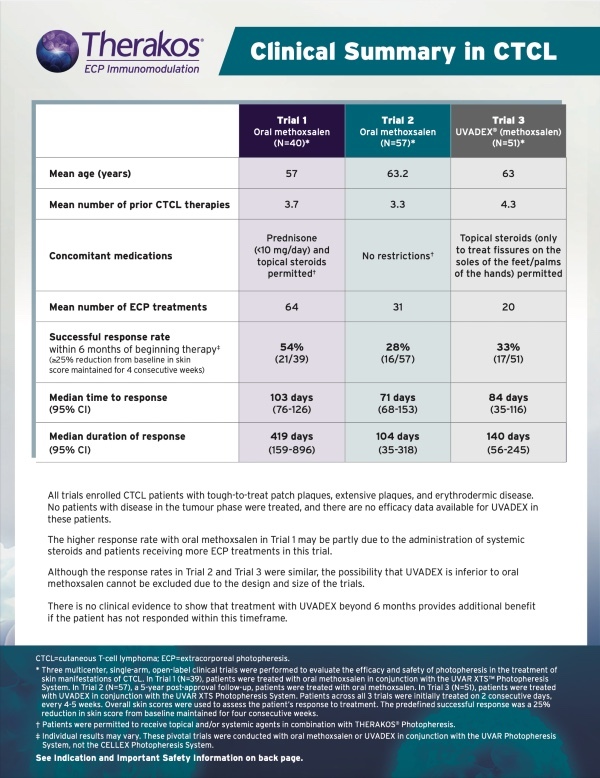
SKIN MANIFESTATIONS OF CUTANEOUS T-CELL LYMPHOMA (CTCL)
MEANINGFUL RESPONSES, EVEN IN A TOUGH—TO—TREAT DISEASE6*
FOR PATIENTS UNRESPONSIVE TO SKIN-DIRECTED TREATMENT FOR CTCL SKIN SYMPTOMS6
THERAKOS PHOTOPHERESIS HARNESSES THE PATIENT’S IMMUNE SYSTEM TO HELP TREAT THE SKIN MANIFESTATIONS OF CTCL5
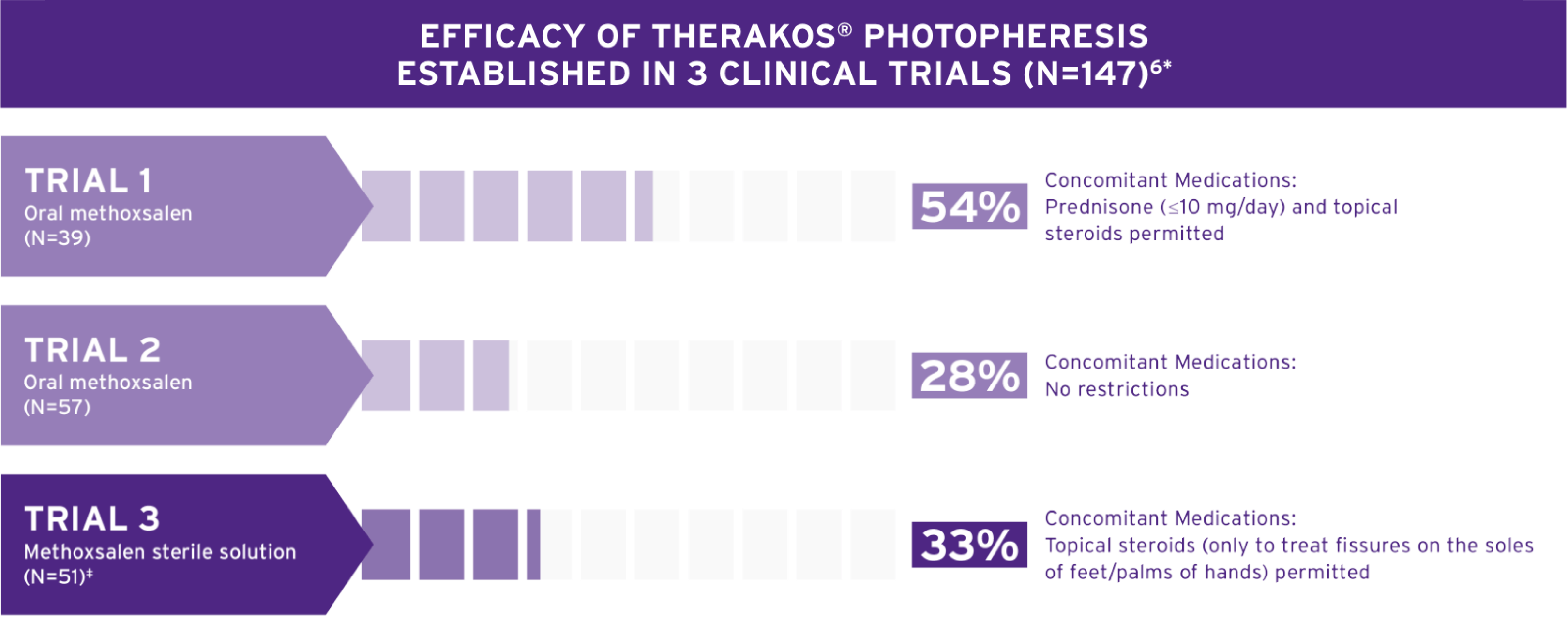

- Individual results may vary. All trials enrolled CTCL patients with tough-to-treat patch plaques, extensive plaques, and erythrodermic disease. Patients were permitted to receive limited topical and/or systematic agents in combination with THERAKOS Photopheresis. Methoxsalen Sterile Solution is indicated for extracorporeal administration with the THERAKOS CELLEX® Photopheresis System in the palliative treatment of the skin manifestations of CTCL that is unresponsive to other forms of treatment.6
* Individual results may vary. All trials enrolled CTCL patients with tough-to-treat patch plaques, extensive plaques, and erythrodermic disease. Patients were permitted to receive limited topical and/or systematic agents in combination with THERAKOS Photopheresis. Methoxsalen Sterile Solution is indicated for extracorporeal administration with the THERAKOS CELLEX® Photopheresis System in the palliative treatment of the skin manifestations of CTCL that is unresponsive to other forms of treatment.6
Three multicenter, single-arm, open-label clinical trials were performed to evaluate the efficacy and safety of photopheresis in the treatment of skin manifestations of CTCL. Overall skin scores were used in the clinical trials of ECP to assess the patient’s response to treatment. The predefined successful response was a 25% reduction in skin score from baseline maintained for four consecutive weeks.6 All patients had CTCL with tough-to-treat patch plaques, extensive plaques, and erythrodermic disease. No patients with the disease in the tumor phase were treated. There are no data available regarding the efficacy of methoxsalen sterile solution in patients with disease in the tumor phase.6
Trial 1 Design (N=39): Treated with the oral formulation of methoxsalen in conjunction with the UVAR XTS Photopheresis System. Prednisone up to 10 mg/day was permitted in addition to topical steroids. Patients were initially treated on two consecutive days, every four to five weeks.6 The specific instrument model used for this study, the UVAR XTS Photopheresis System, is no longer manufactured or supported, and has been replaced by the THERAKOS CELLEX Photopheresis System.
Trial 2 Design (N=57): Five-year post-approval follow up of CTCL patients conducted to evaluate long-term safety. This study used the oral dosage formulation of methoxsalen. There was no concomitant medication restriction. Patients were initially treated on two consecutive days, every four to five weeks.6
Trial 3 Design (N=51): Patients were treated with methoxsalen sterile solution in conjunction with the UVAR XTS Photopheresis System. The total dose of methoxsalen sterile solution delivered was substantially lower (approximately 200 times) than that used with oral administration. Topical steroids were permitted only for the treatment of fissures on the soles of the feet and the palms of the hands. All other steroids, topical or systemic, were prohibited. Patients were initially treated on two consecutive days, every four to five weeks. 15 of 17 responses were seen within six months of treatment. Only 2 patients responded to treatment after 6 months.6 The specific instrument model used for this study, the UVAR XTS Photopheresis System, is no longer manufactured or supported, and has been replaced by the THERAKOS CELLEX Photopheresis System.
- Although the response rates with methoxsalen sterile solution in Trial 3 and oral methoxsalen in Trial 2 were similar, the possibility that methoxsalen sterile solution is inferior in efficacy to oral methoxsalen cannot be excluded due to the design and size of the clinical trials6
- The higher response rate with oral methoxsalen in Trial 1 may be partly due to the coadministration of systemic steroids and patients receiving more ECP treatments (Mean number of treatments: Trial 1=64, Trial 2=31, Trial 3=20)6
PATIENTS WITH THE SKIN MANIFESTATIONS OF CTCL SHOULD CONTINUE TREATMENT FOR A MINIMUM OF 7 CYCLES6
- All but 2 responses were achieved within 6 months of treatment in Trial 3
- There is no clinical evidence of additional benefit from treatment with methoxsalen sterile solution beyond 6 months or a different schedule
WELL-TOLERATED THERAPY THAT HELPS MANAGE THE SKIN MANIFESTATIONS OF CTCL6*
- Adverse events (AEs) in clinical trials were primarily related to hypotension secondary to changes in extracorporeal volume (>1%)
- In Trial 3:
- Six serious cardiovascular AEs (5 unrelated to photopheresis) were reported in 5 patients (10%)
- Six infections were also reported in 5 patients
- Two were Hickman catheter infections in 1 patient
- The most common post-marketing events reported with methoxsalen sterile solution/ECP treatment regardless of causality assessment were obtained from spontaneous reports, clinical studies or literature and include taste perversions, vasovagal attack/fainting/dizziness, sepsis/line sepsis, anemia, hypotension, and nausea
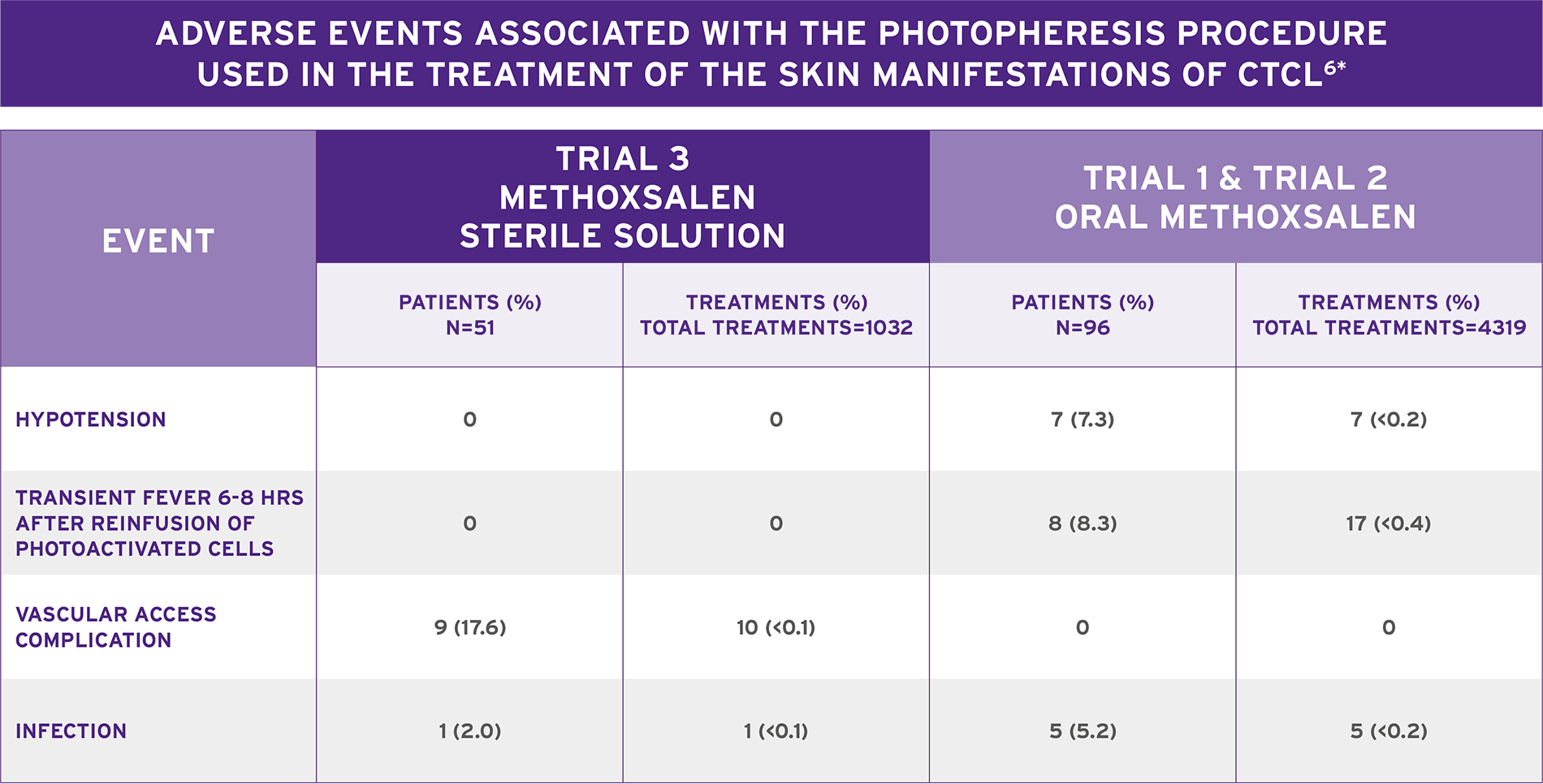
*Methoxsalen Sterile Solution is indicated for extracorporeal administration with the THERAKOS® UVAR XTS™ or THERAKOS CELLEX® Photopheresis System in the palliative treatment of the skin manifestations of CTCL that is unresponsive to other forms of treatment.6
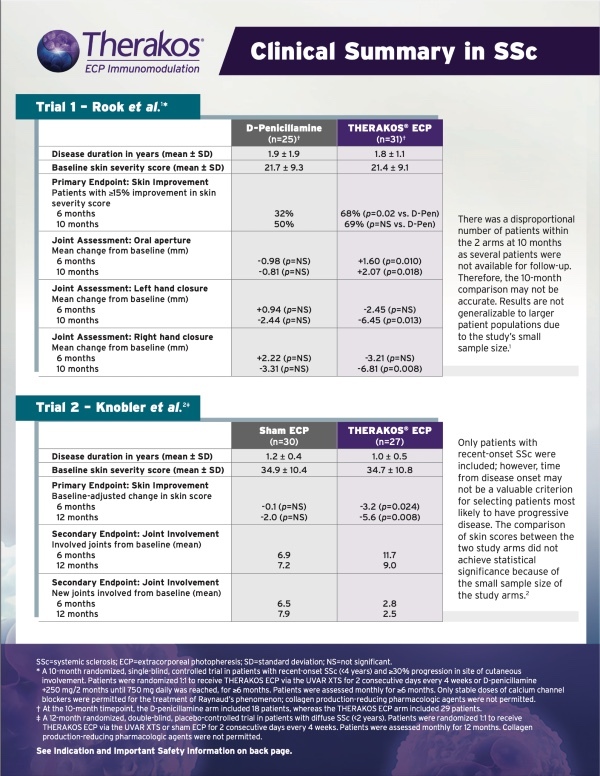
SYSTEMIC SCLEROSIS (SSc)
STATISTICALLY SIGNIFICANT IMPROVEMENT IN SKIN SCORE AFTER 6 MONTHS7*
FOR PATIENTS WITH EARLY-ONSET, DIFFUSE SYSTEMIC SCLEROSIS7
THERAKOS PHOTOPHERESIS HARNESSES THE PATIENT’S IMMUNE SYSTEM TO HELP TREAT SYSTEMIC SCLEROSIS5
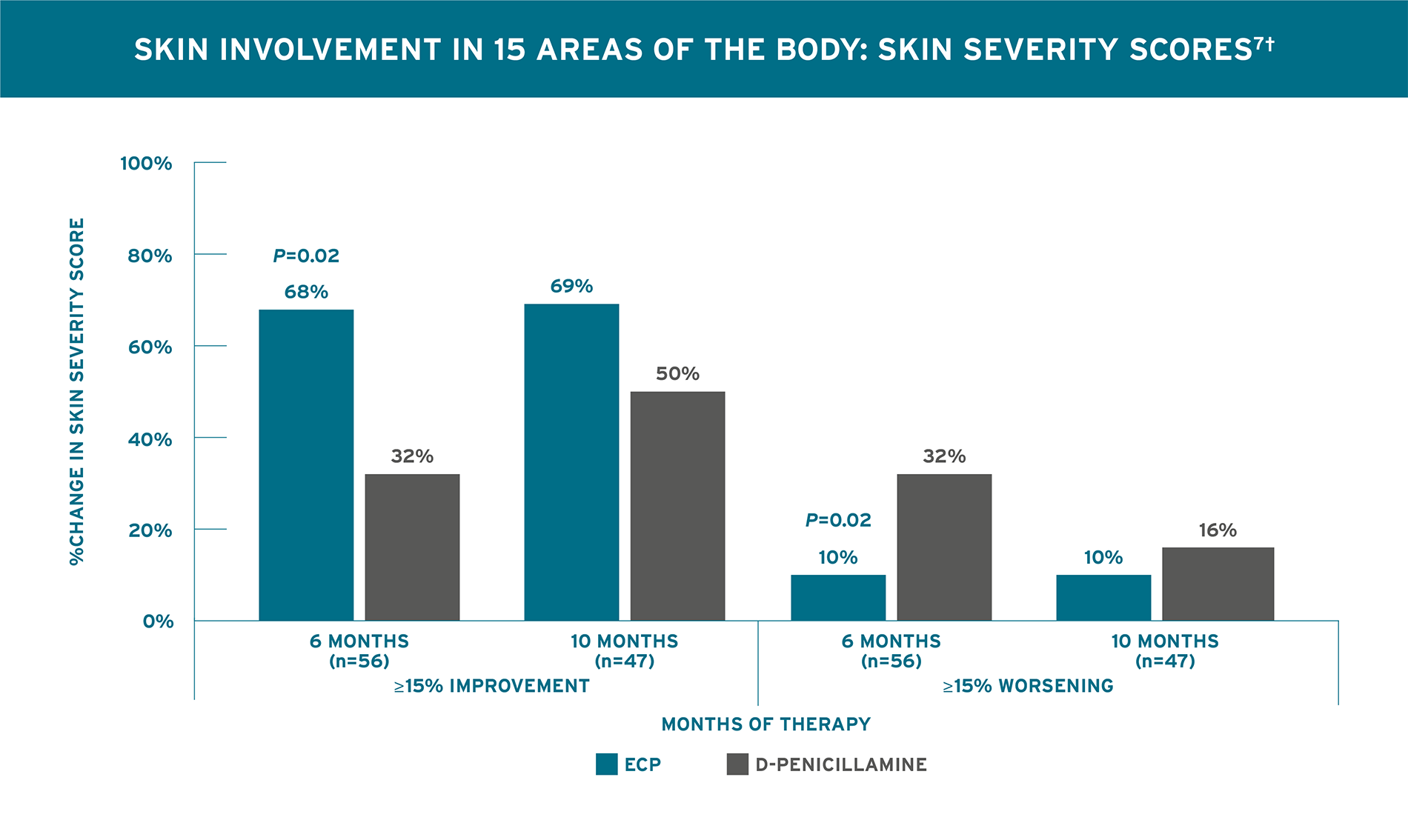
Rook 1992 Trial Design (N=56): A 10-month randomized, single-blind, controlled trial in patients with recent onset systemic sclerosis. Outcomes included skin score assessed by rating the thickness of the skin on a 0 to 3 scale in 15 areas of the body with possible skin severity scores ranging from 0 to 45 and joints assessed by standardized measurement of the change in oral aperture and right and left-hand closure. All patients had recent onset systemic sclerosis (<4 years duration) and ≥30% progression in site of cutaneous involvement. Patients were randomized 1:1 to receive ECP, 2 consecutive days, every 4 weeks (n=31) or D-Penicillamine (n=25), 750 mg/day (the dose was increased by 250 mg every two months until a daily dose of 750 mg/day was reached). Patients were assessed monthly for a minimum duration of 6 months. Collagen production-reducing pharmacologic agents like steroids, colchicine, potassium aminobenzoate, and griseofulvin were not permitted during the study. For treatment of Raynaud’s phenomenon, only stable doses of calcium channel blockers were permitted.7
*No significant improvement in skin score of ECP compared to D-Pen-treated patients was observed at 10 months.7 †A change in the skin score during treatment was considered to be clinically relevant if it differed by at least 15% from baseline.7
SIGNIFICANT IMPROVEMENT COMPARED TO BASELINE IN ORAL APERTURE AFTER 6 MONTHS AND BOTH ORAL APERTURE AND RIGHT/LEFT-HAND CLOSURE AFTER 10 MONTHS7
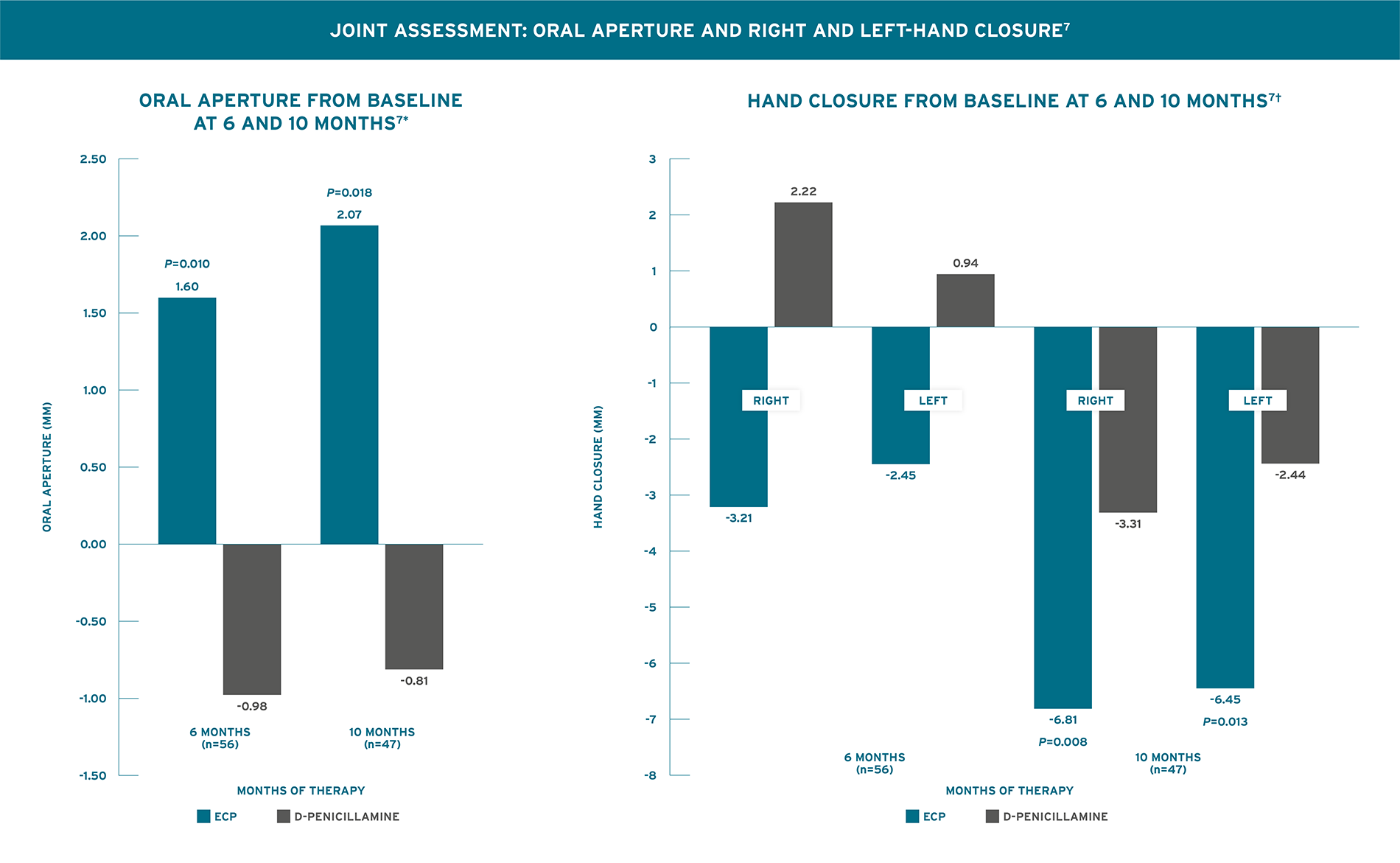
Rook 1992 Trial Design (N=56): A 10-month randomized, single-blind, controlled trial in patients with recent onset systemic sclerosis. Outcomes included skin score assessed by rating the thickness of the skin on a 0 to 3 scale in 15 areas of the body with possible skin severity scores ranging from 0 to 45 and joints assessed by standardized measurement of the change in oral aperture and right and left-hand closure. All patients had recent onset systemic sclerosis (<4 years duration) and ≥30% progression in site of cutaneous involvement. Patients were randomized 1:1 to receive ECP, 2 consecutive days, every 4 weeks (n=31) or D-Penicillamine (n=25), 750 mg/day (the dose was increased by 250 mg every two months until a daily dose of 750 mg/day was reached). Patients were assessed monthly for a minimum duration of 6 months. Collagen production-reducing pharmacologic agents like steroids, colchicine, potassium aminobenzoate, and griseofulvin were not permitted during the study. For treatment of Raynaud’s phenomenon, only stable doses of calcium channel blockers were permitted.7
*No significant improvement in oral aperture of D-pen-treated patients compared to baseline was observed.7 †No significant improvement in hand closure of ECP-treated patients compared to baseline was observed at 6 months. No significant improvement in hand closure of D-Pen-treated patients compared to baseline was observed.7
STATISTICALLY SIGNIFICANT SKIN IMPROVEMENT FROM BASELINE AT 6 AND 12 MONTHS8
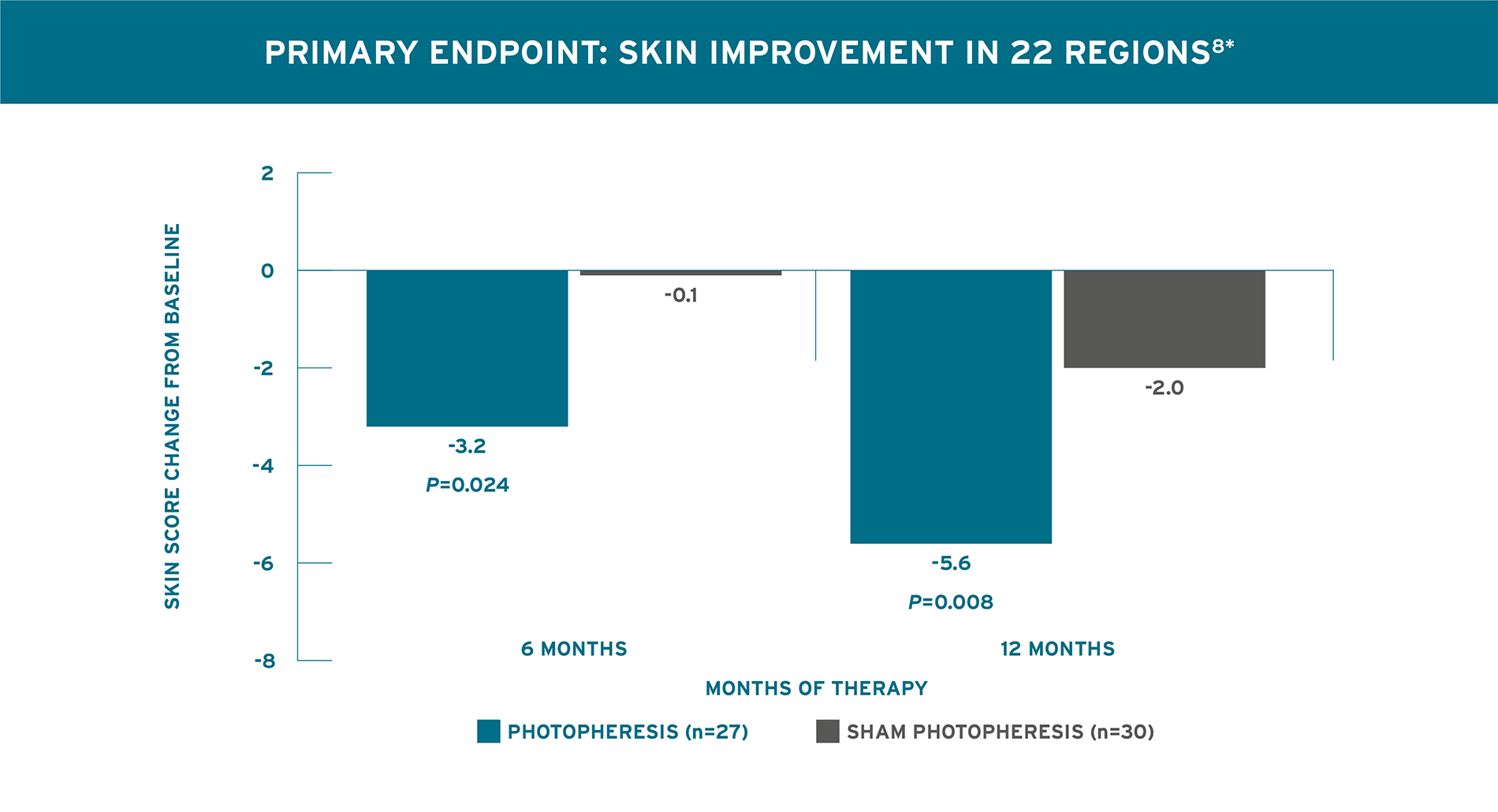
Knobler 2006 Trial Design (N=57): A 12-month randomized, double-blind, placebo-controlled trial in patients with diffuse systemic sclerosis. The primary endpoint was decrease in skin involvement evaluated by skin score in 22 regions with the modified scleroderma skin scoring method recommended by Kahaleh et al, Clin Exp Rheumatol 1986;4:367-369. The secondary endpoint was change in joint involvement as measured with a goniometer. All patients had diffuse systemic sclerosis and were <2 years from disease onset. Patients were randomized 1:1 to receive Photopheresis, 2 consecutive days, every 4 weeks (n=27) or Sham Photopheresis, 2 consecutive days, every 4 weeks (n=30). Patients were assessed monthly for 12 months. Collagen production-reducing pharmacologic agents, including corticosteroids, colchicine, griseofulvin, and D-Pen were not permitted.8
*Comparison of skin scores between the two study arms did not achieve statistical significance because of the small sample size of the study arms.8
STATISTICALLY SIGNIFICANT IMPROVEMENT FROM BASELINE IN A GREATER NUMBER OF JOINTS AND HAD FEWER NEW JOINTS BECOME INVOLVED AFTER 6 AND 12 MONTHS8
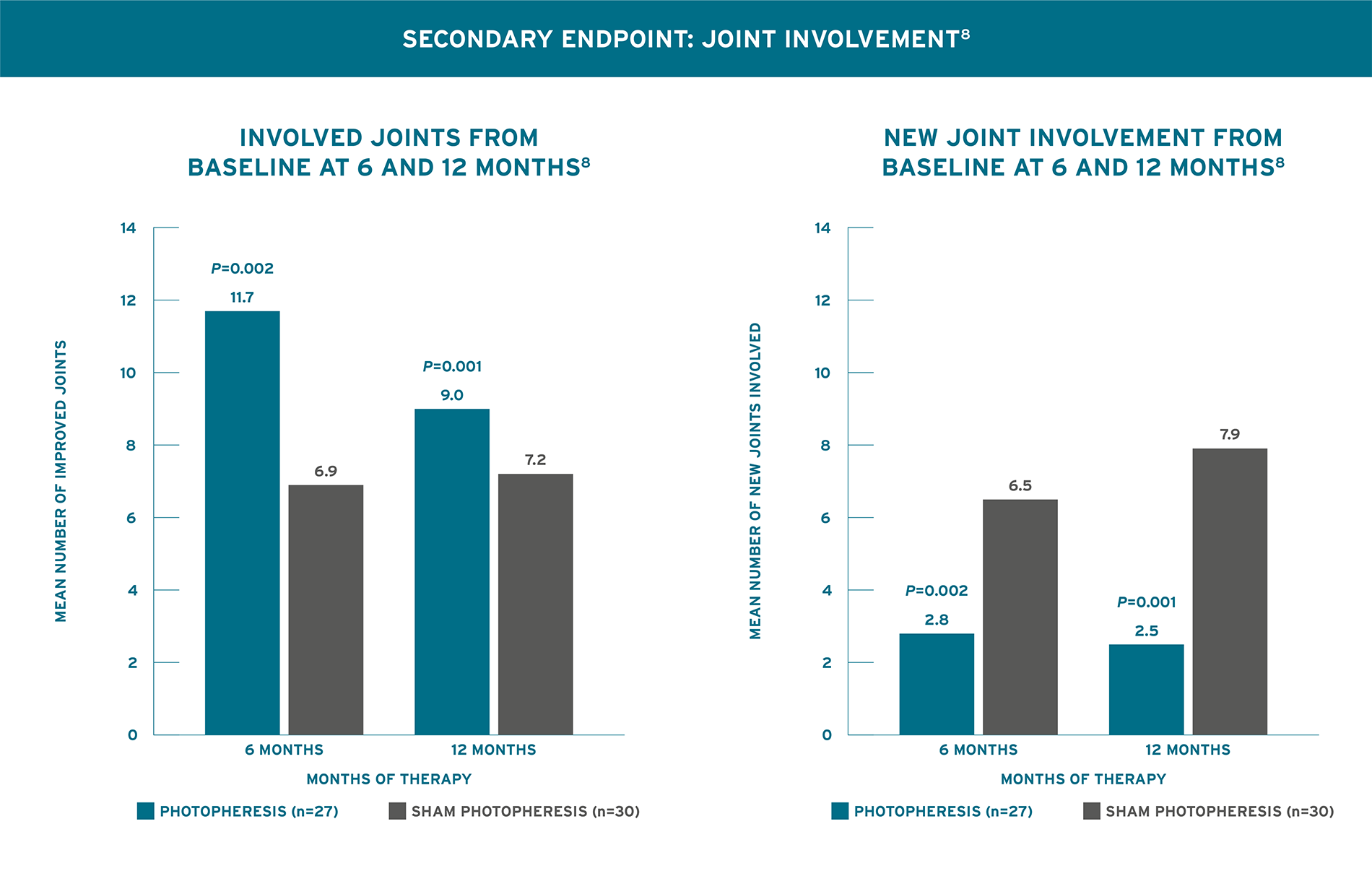
Knobler 2006 Trial Design (N=57): A 12-month randomized, double-blind, placebo-controlled trial in patients with diffuse systemic sclerosis. The primary endpoint was decrease in skin involvement evaluated by skin score in 22 regions with the modified scleroderma skin scoring method recommended by Kahaleh et al, Clin Exp Rheumatol 1986;4:367-369. The secondary endpoint was change in joint involvement as measured with a goniometer. All patients had diffuse systemic sclerosis and were <2 years from disease onset. Patients were randomized 1:1 to receive Photopheresis, 2 consecutive days, every 4 weeks (n=27) or Sham Photopheresis, 2 consecutive days, every 4 weeks (n=30). Patients were assessed monthly for 12 months. Collagen production-reducing pharmacologic agents, including corticosteroids, colchicine, griseofulvin, and D-Pen were not permitted.8
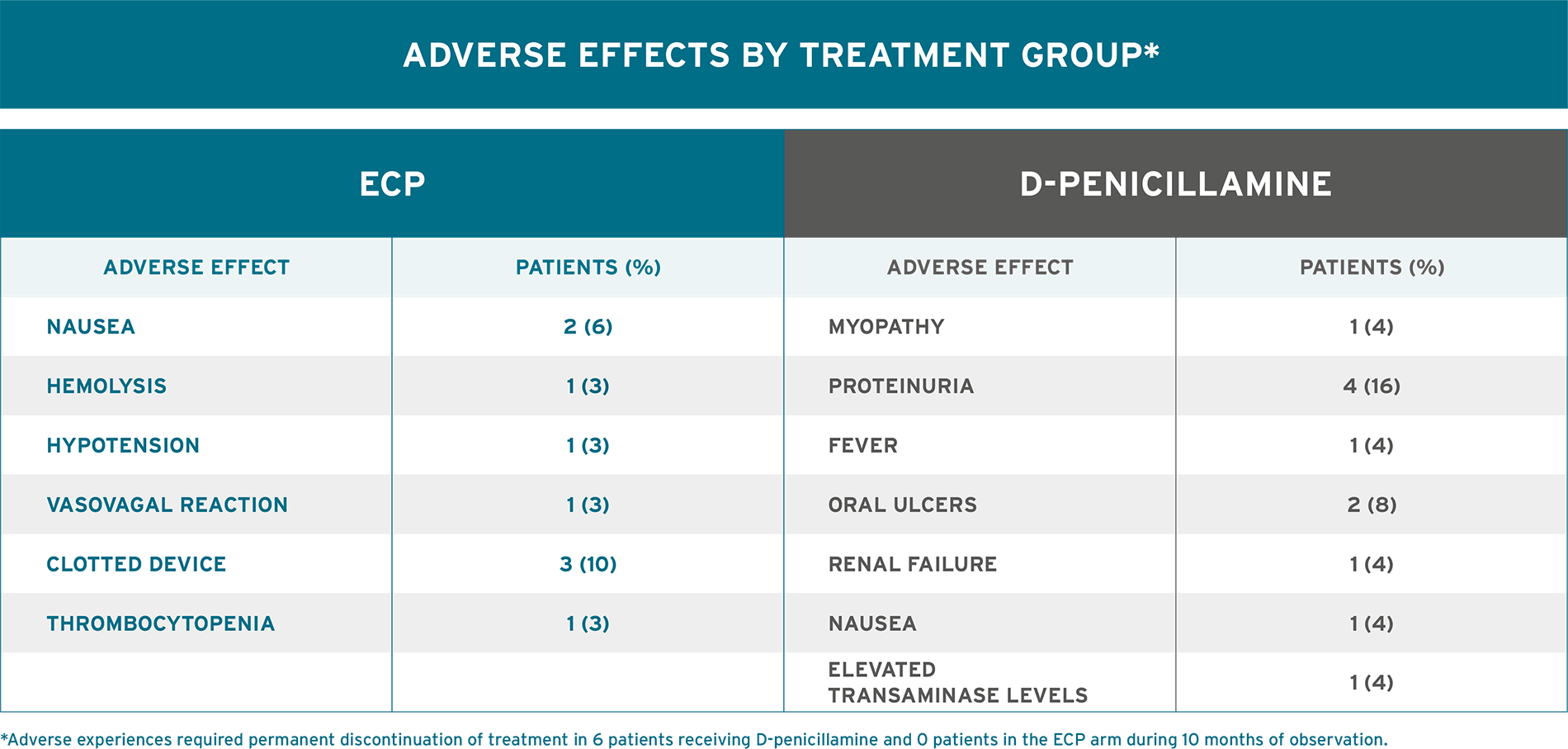
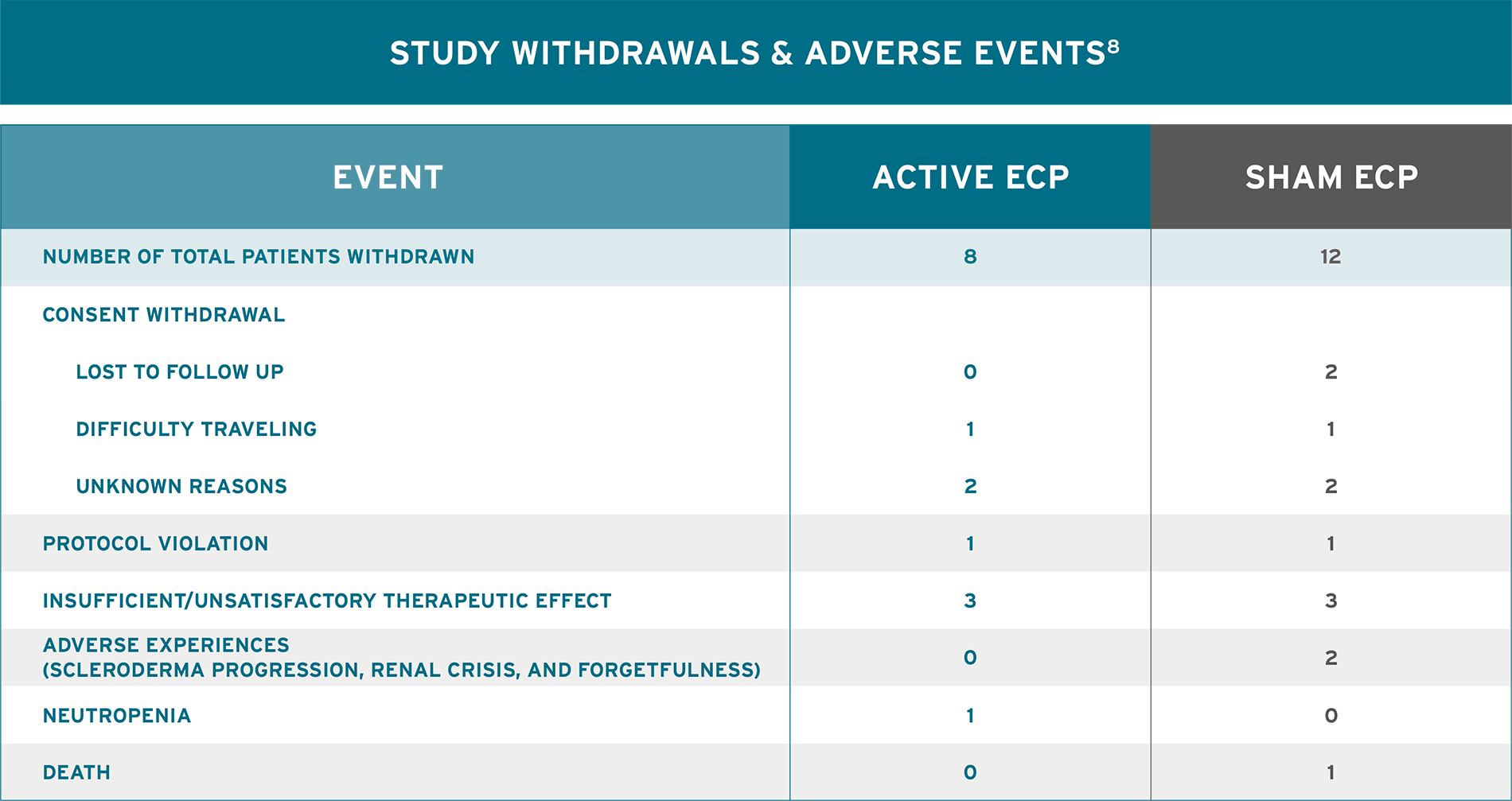
WELL-TOLERATED TREATMENT MODALITY THAT CAN HELP MANAGE SKIN MANIFESTATIONS OF SYSTEMIC SCLEROSIS8
AN ESTABLISHED TOLERABILITY PROFILE8
- No serious adverse events and no significant difference in overall adverse events between study arms were reported
THERAKOS® CELLEX® PHOTOPHERESIS SYSTEM
DESIGNED FOR EXCELLENCE IN ECP
THE CELLEX SYSTEM IS A FULLY INTEGRATED AND VALIDATED ECP SYSTEM, DESIGNED TO HELP MEET THE NEEDS OF BOTH YOU AND YOUR PATIENTS.3

EXPERIENCE & CERTIFICATION
DEVELOPING ECP SOLUTIONS (PRODUCTS, SERVICES, AND EDUCATION) SINCE 19873
THERAKOS® CELLEX® PHOTOPHERESIS SYSTEMS HAVE A WORLDWIDE REACH9

Used by >300 treatment centres

In >30 countries

ECP kits currently being provided for >135,000 patients per year

>2 million treatments in over 35 years
THERAKOS CELLEX PHOTOPHERESIS meets the following global quality standards:
- ISO 13485:2003 certified9*
- THERAKOS Quality Management System certified for the design, manufacture, distribution, and service of photopheresis systems
- European Council Declaration of Conformity10
- Medical Device Directive 93/42/EEC9,10 (UK specific)
- Satisfies requirement that the devices can be used safely with the materials, substances, and gases with which they enter into contact
- Satisfies requirement that performance is maintained in accordance with their intended use
*Certified by a Notified Body (BSI Certificate of Registration number FM 72801).9 The International Organization for Standardization (ISO) specifies requirements for a quality management system (QMS). Organizations use ISO standards to demonstrate their ability to consistently provide products and services that meet customer and regulatory requirements.11 ISO 13485:2003 specifies requirements for a QMS for medical devices and related services.12
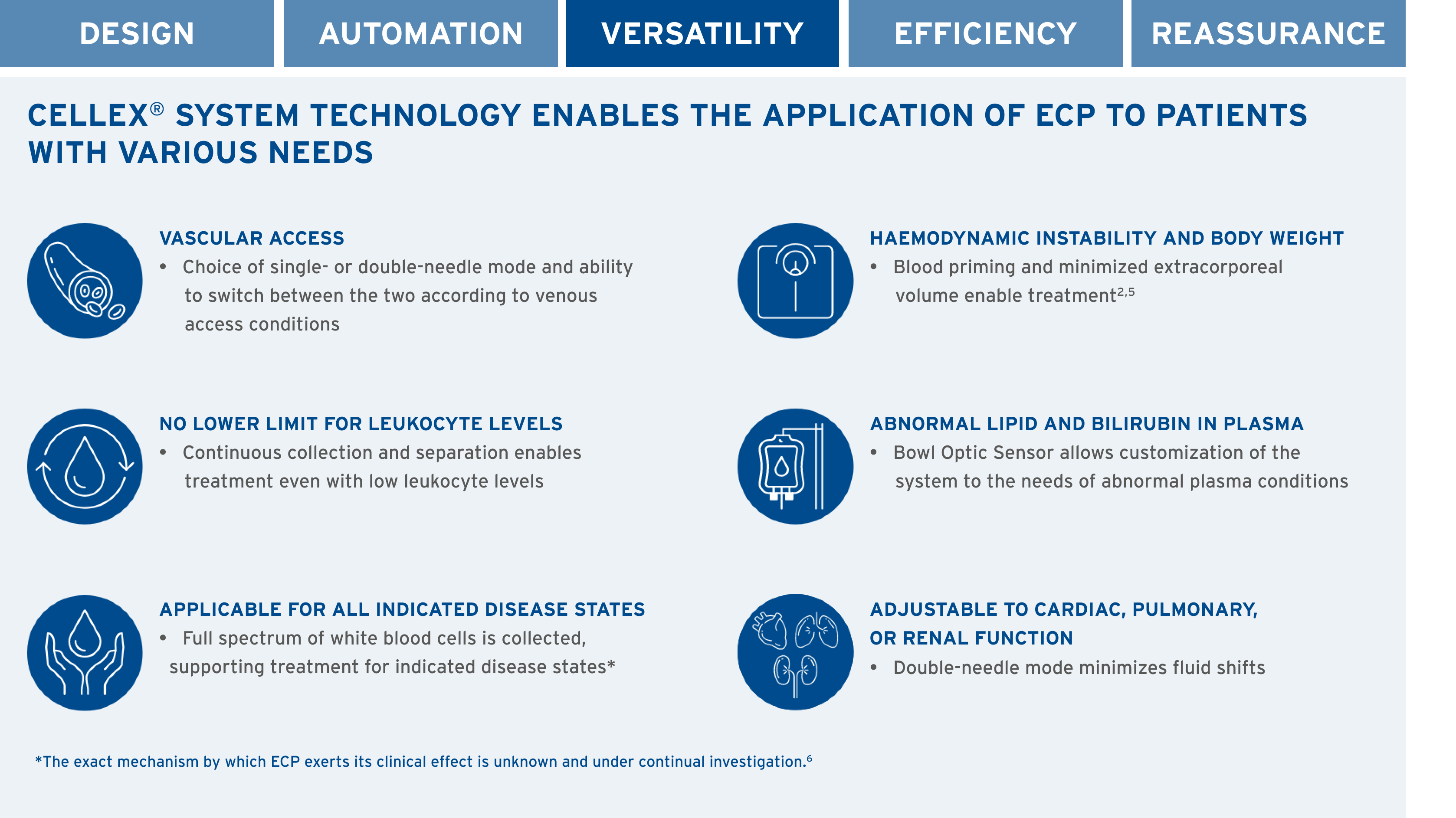
SELECT SYSTEM FEATURES
CANADA’S ONLY FULLY INTEGRATED ECP SYSTEM3




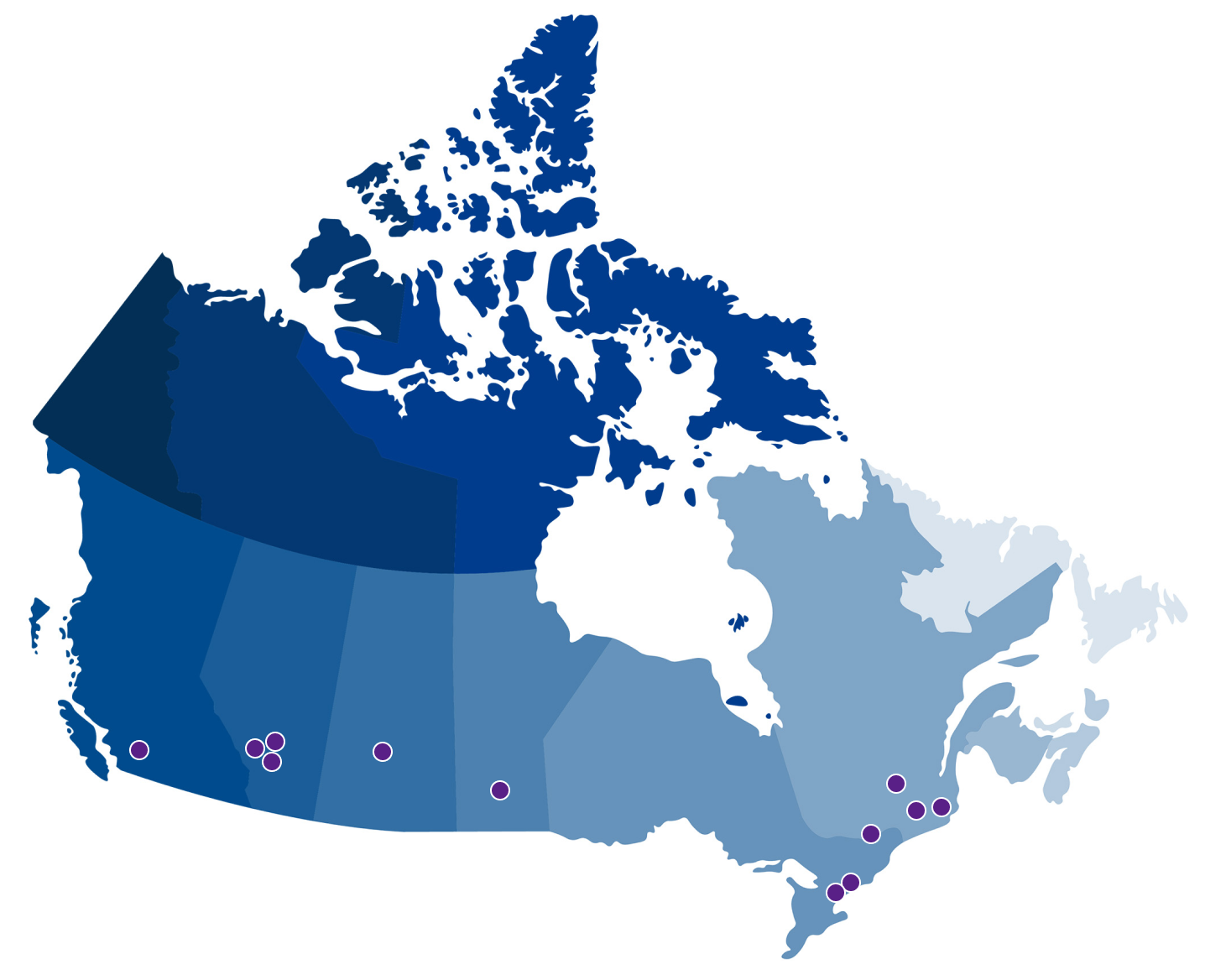
AVAILABILITY—ECP TREATMENT CENTRES
There is a growing footprint of centres that offer THERAKOS Photopheresis treatment across Canada. These treatment centres are independent, third-party facilities not owned or operated by Therakos (Canada) Company. The treatment centre information provided is for informational use only, is subject to change, and may not be comprehensive.
TO GET INFORMATION ABOUT CONTACTING THE ECP DIRECTOR AT A PARTICULAR TREATMENT CENTRE, PLEASE FILL OUT A GENERAL INQUIRY FORM WITH YOUR REQUEST.
RESOURCES
PROFESSIONAL RESOURCES
DOWNLOADABLE BROCHURES
VIDEOS


WATCH THERAKOS CELLEX PHOTOPHERESIS PATIENT PROCEDURE VIDEO
Watch the video to understand the THERAKOS CELLEX Photopheresis System ECP Immunomodulation procedure.
THERAKOS ONE TEAM CUSTOMER SUPPORT
OUR DEDICATED IN-HOUSE SUPPORT TEAM WILL HELP ENSURE OPTIMAL DELIVERY OF ECP TREATMENTS TO YOUR PATIENTS. WITH ALL OF OUR LEARNING INITIATIVES, WE AIM TO PROVIDE THE HIGHEST STANDARDS AND CONTRIBUTE TO THE CREATION OF A MORE INFORMED MEDICAL COMMUNITY, WHICH IS BETTER PLACED TO DELIVER ECP IMMUNOMODULATION TO PATIENTS.
THERAKOS ONE TEAM SOLUTIONS
- Operational training & troubleshooting
- Machine repair & servicing*
- Order taking & delivery
- Contract options & adaptations

Fully integrated team enables more quality oversight and overall improved service

Bilingual hotline support available
- Customer Service
- Technical & Clinical Support

Fast turnaround times for repairs
- 24 hours for critical repairs*
- 48 hours for non-critical repairs†
References:
- Therakos, Inc. Operator’s Manual. THERAKOS® CELLEX® Photopheresis System.
- Bisaccia E, et al. Br J Dermatol. 2009;161(1):167-169.
- Knobler R, et al. J Eur Acad Dermatol Venereol. 2014;28(suppl 1):1-37.
- Perotti C, Sniecinski I. Transfus Apher Sci. 2015;52:360-368.
- Hart JW, et al. Ther Adv Hematol. 2013;4:320-334.
- UVADEX (methoxsalen) Product Monograph. West Chester, PA, USA: Therakos, Inc.: May 2013.
- Rook AH, et al. Arch Dermatol. 1992;128:337-346.
- Knobler R, et al. J Am Acad Dermatol. 2006;54:793-799.
- Therakos (Canada) Company. Data on File. February 2021.
- European Council. Council Directive 93/42/EEC. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31993L0042&from=EN. Accessed February 9, 2021.
- International Organization for Standardization. ISO 9001:2015 Quality Management Systems – Requirements. Published online September 2019. https://www.iso.org/standard/62085.html. Accessed March 16, 2021.
- International Organization for Standardization. ISO 13485:2016 Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes. Published online March 2016. https://www.iso.org/standard/59752.html. Accessed May 27, 2025.
References:
- Knobler R, et al. J Eur Acad Dermatol Venereol. 2014;28(suppl 1):1-37.
- Health Canada Medical Devices Active Licence Listing Database. https://health-products.canada.ca/mdall-limh/information.do?companyId_idCompanie=129103&lang=eng. Accessed May 27, 2021.
- Therakos LLC. Data on File. February 2021.
References:
- Therakos, Inc. Operator’s Manual. THERAKOS™ CELLEX™ Photopheresis System.
- Bisaccia E, et al. Br J Dermatol. 2009;161(1):167-169.
- Knobler R, et al. J Eur Acad Dermatol Venereol. 2014;28(suppl 1):1-37.
- Perotti C, Sniecinski I. Transfus Apher Sci. 2015;52(3):360-368.
- Hart JW, et al. Ther Adv Hematol. 2013;4:320-334.
- UVADEX (methoxsalen) Product Monograph. West Chester, PA, USA: Therakos, Inc.: May 2013.
- Rook AH, et al. Arch Dermatol. 1992;128(3):337-346.
- Knobler R, et al. J Am Acad Dermatol. 2006;54:793-799.
- Therakos LLC. Data on File. February 2021.
- European Council. Council Directive 93/42/EEC. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31993L0042&from=EN. Accessed February 9, 2021.
- International Organization for Standardization. ISO 9001:2015 Quality Management Systems – Requirements. Published online September 2019. https://www.iso.org/standard/62085.html. Accessed March 16, 2021.
- International Organization for Standardization. ISO 13485:2003 Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes. Published online July 2003. https://www.iso.org/standard/36786.html. Accessed March 16, 2021.