OUR LEGACY
PIONEERING ECP IMMUNOMODULATION FOR OVER 35 YEARS1
THERAKOS® CELLEX® Photopheresis focuses on the delivery of extracorporeal photopheresis (ECP) immunomodulation. We provide products and services aimed at advancing the science and clinical practice of the therapy and are committed to doing everything we can to help provide optimal delivery of ECP immunomodulation for both healthcare professionals (HCPs) and patients.

35 YEARS
We have a more than 35-year history in the field of ECP and, as such, have solid expertise and experience, as well as multi-disciplinary personnel who are available to respond to various treatment related customer needs.1
INTEGRITY
We do the right thing, and we can be trusted to align our actions and our words with our mission and values.


COLLABORATIVE
We own it, together. Not only do we hold ourselves and each other accountable for our shared success, we are inclusive and always work together towards our common goals.
INNOVATIVE
We innovate to help our patients thrive. By thinking differently, we solve complex challenges with innovative solutions, and we are always seeking new ways to continuously improve our performance.


PATIENT-CENTRIC
We always put our patients and their families first because they are at the heart of what we do. Our decisions and our actions are guided by our commitment to improve lives.
DEDICATED TO THE PRACTICE AND TECHNOLOGICAL ADVANCEMENT OF ECP FOR OVER 35 YEARS1
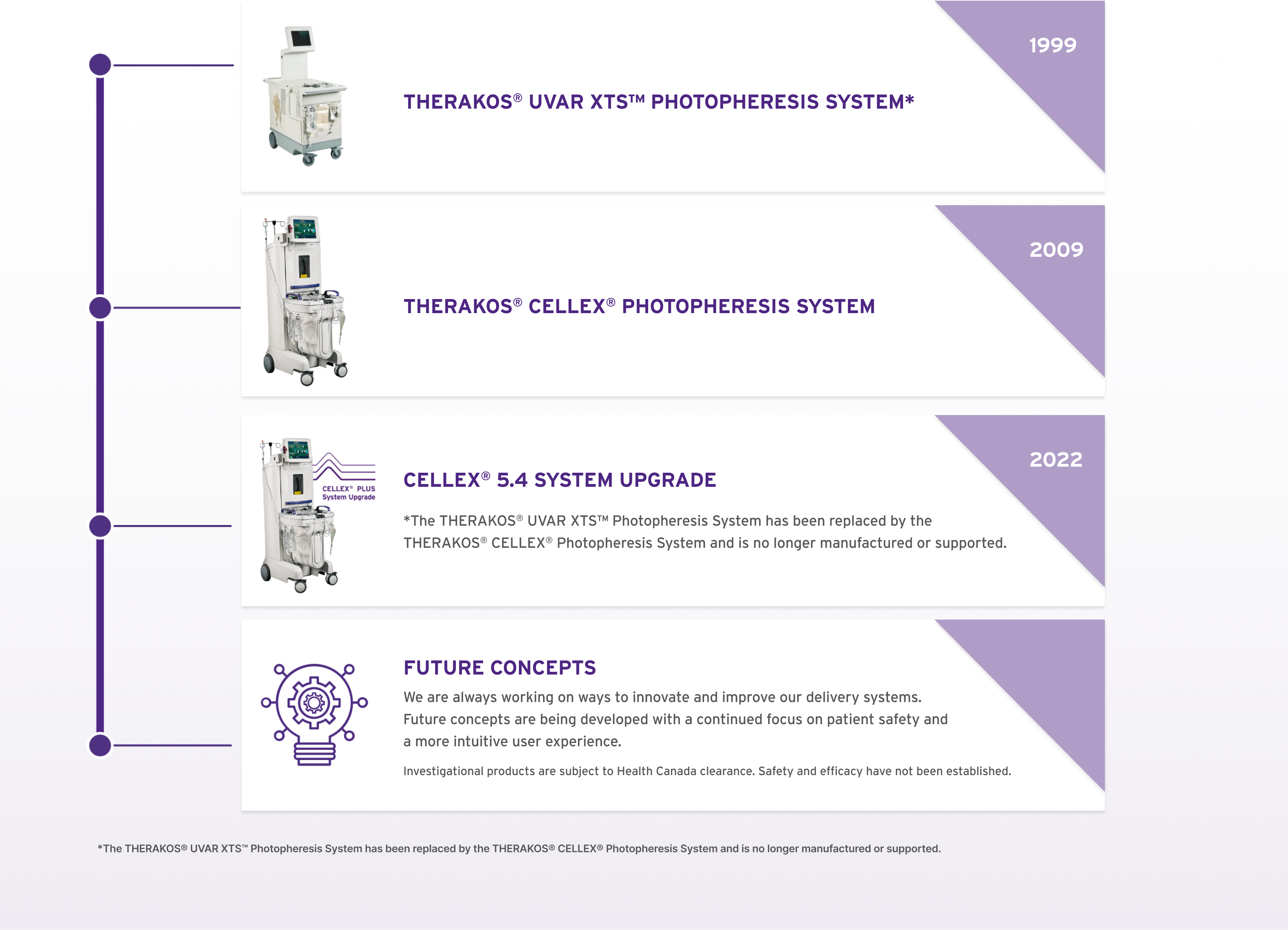
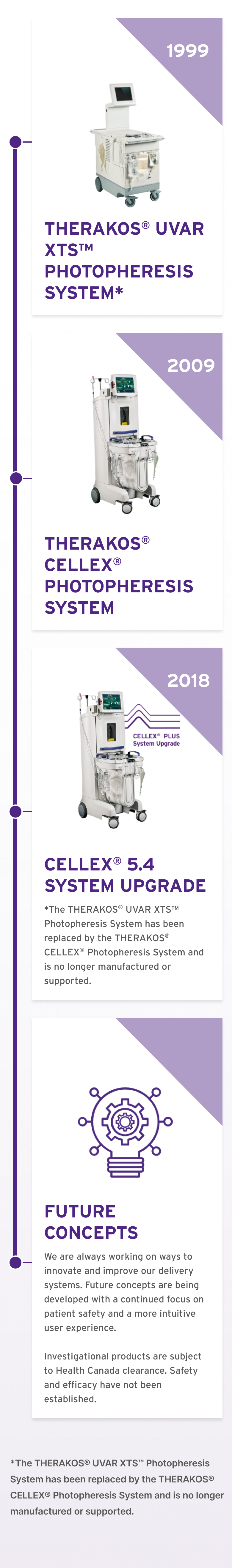
We have been pioneering ECP since 1988 through the evolution of our integrated ECP immunomodulation platforms with the first system available for use in Canada in 1999.1,2* Since 2009, the THERAKOS® CELLEX® Photopheresis System has been used to deliver over 2 million treatments and is used in over 30 countries.1
ECP IMMUNOMODULATION PLATFORMS IN CANADA
References:
- Knobler R, et al. J Eur Acad Dermatol Venereol. 2014;28(suppl 1):1-37.
- Health Canada Medical Devices Active Licence Listing database. https://health-products.canada.ca/mdall-limh/information?licenceId=7703&type=active&lang=eng. Accessed May 27, 2025.
- Therakos (Canada) Company. Data on File. February 2021.
References:
- Knobler R, et al. J Eur Acad Dermatol Venereol. 2014;28(suppl 1):1-37.
- Health Canada Medical Devices Active Licence Listing Database. https://health-products.canada.ca/mdall-limh/information.do?companyId_idCompanie=129103&lang=eng. Accessed May 27, 2021.
- Therakos LLC. Data on File. February 2021.
References:
- Therakos, Inc. Operator’s Manual. THERAKOS™ CELLEX™ Photopheresis System.
- Bisaccia E, et al. Br J Dermatol. 2009;161(1):167-169.
- Knobler R, et al. J Eur Acad Dermatol Venereol. 2014;28(suppl 1):1-37.
- Perotti C, Sniecinski I. Transfus Apher Sci. 2015;52(3):360-368.
- Hart JW, et al. Ther Adv Hematol. 2013;4:320-334.
- UVADEX (methoxsalen) Product Monograph. West Chester, PA, USA: Therakos, Inc.: May 2013.
- Rook AH, et al. Arch Dermatol. 1992;128(3):337-346.
- Knobler R, et al. J Am Acad Dermatol. 2006;54:793-799.
- Therakos LLC. Data on File. February 2021.
- European Council. Council Directive 93/42/EEC. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31993L0042&from=EN. Accessed February 9, 2021.
- International Organization for Standardization. ISO 9001:2015 Quality Management Systems – Requirements. Published online September 2019. https://www.iso.org/standard/62085.html. Accessed March 16, 2021.
- International Organization for Standardization. ISO 13485:2003 Medical Devices – Quality Management Systems – Requirements for Regulatory Purposes. Published online July 2003. https://www.iso.org/standard/36786.html. Accessed March 16, 2021.



